INCONCRETO NEWS
From Hippocrates to Cell and Gene Therapy – Part III
The CGT value chain: differences from the traditional pharma
The development in Cell & Gene Therapy (CGT) – deeply accelerated since the beginning of the Covid-19 pandemic – is leading to a major shift of paradigm in the biopharmaceutical business model.
CGT represents unique opportunities for care, opening the way to unprecedented and unthinkable cures and provoking a positive impact on the global society.
In the meantime, such change cannot happen without crucial interventions on the entire value chain of CGT, which must be founded on different operating principles. Traditional pharma is based on pills and drugs, which are produced according to standardized procedures. On the other side, personalized medicines – and CGT especially – must imperatively be tailored to patients, to their specificities and needs.

These differences mean that the entire cycle of CGT is facing various challenges.
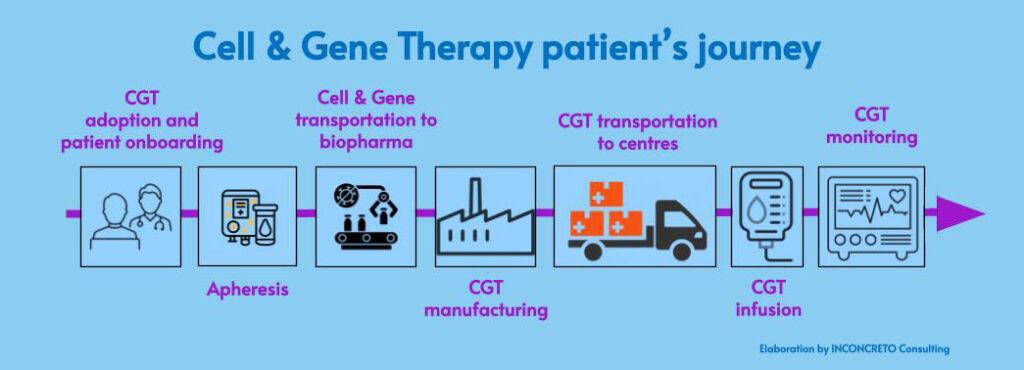
- Defining the ‘right’ patients for CGT. CGT CGT can be applied to patients once ad hoc trials have been successfully conducted and concluded. However, due to the specificities of CGT itself, it becomes problematic to find qualified clinical trial subjects and subsequently commercial patients. Dealing with tailor-made therapies, the risk of failing the enrollment targets increases.
- Establishing dedicated centres for CGT. In light of the large amount of personalized research required for CGT, the go-to-market model needs to be preceded by the identification of centralized sites of care at the regional level. These specialized centres are the places where identified patients, after receiving treatment recommendations, start the CGT procedures, the first phase being apheresis, i.e. when the cells are extracted from patients.
- Orchestrating the supply chain. The high level of uncertainties and asymmetries within CGT requires the necessary adaptation of manufacturing to all the phases in the supply chain. For on-demand and just-in-time operations to be successful, robust logistics and asset monitoring capabilities are essential. It is particularly essential to align demand and production with the delivery and reinfusion of patient-specific doses in CGT care centres. Finally, continuous monitoring, and assessing the effectiveness of CGT is crucial for patients.
- Optimizing and scaling up. CGT can be progressively optimized and scaled up at a higher level by companies willing to meet the needs of the patients. Different possibilities for deploying larger businesses are conceivable. This is due to the creation of an independent CGT unit for all relevant commercial functions across the markets. It is also from fully integrating the CGT business into the central footprint, including comprehensive CGT resources within each function.
Making sure that the benefits of CGT are directed to the largest public possible is an absolute priority. Also from an economic perspective, the successful conception and implementation of new CGT launches is an asset primarily for patients, but also for healthcare providers and the distributors of such therapies.
The role of digital in CGT: optimization, interconnection and new challenges
The key to this success can be unveiled and facilitated by the increasingly integrated use of digital technology at each step of the CGT value chain.
Nothing prevents technology from becoming even more at the core of biopharma manufacturing. Digital tools can truly make a difference in CGT: they can provide track-and-trace data on critical information (i.e. the main characteristics of cells extracted during apheresis), better define the timing for treatment delivery and automate the processes.
As a recent study conducted by McKinsey & Company [2022] states, some examples are particularly telling:
- The adoption of digital twins – real-time, virtual versions of physical objects and processes – can enable biopharma companies to optimally balance short- and long-term demand with the supply of raw materials, equipment capacity, and human resources. This double and parallel model facilitates the scheduling of production and lab activities.
- Automation and advanced production technologies such as line sensing, augmented- and assisted-reality tools, and parametric product releases, can allow human operators to make remarkable gains in productivity.
- Predictive analytics can enable real-time monitoring and management of maintenance, environmental, quality, and other supply-chain risks to maximize throughput, cost, compliance, and sustainability.
- Interconnected systems can ensure real-time tracking of site performance, product status, and issue detection, resulting in more effective decision-making by management.
Furthermore – and quite significantly – digitalization can progressively characterize the relationship between the patient and the biopharma ecosystem. When it comes to CGT, where therapies and medicines are deeply personalized according to each patient, digital therapeutics take the form of software, mobile solutions, and AI applications. They also enable remote monitoring through digital devices.
This situation leads to a revolutionary scenario, where big data on diseases and therapies could become even more key than manufacturing itself. Regulators worldwide are starting to deal with the various implications, defining rules for the collection and storage of data, their management and use, and their protection.
A new era in biopharma has started!
INCONCRETO, as an international consultancy, can provide expertise in capital project optimization and oversee critical paths for biopharmaceutical project execution.
Connect with our team! We combine technical expertise with large program execution practices, improving predictable outcomes and steering profitability on Capex/Opex project investments.
For further readings, you may consult these sources:
- Eight imperatives for launching cell and gene therapies, by McKinsey & Company
- How Cell And Gene Therapy Is Transforming Healthcare, by cellandgene.com
- Cell and Gene Therapy Logistics: Meeting the Supply Chain Challenge, by eureka
- Reimagining the future of biopharma manufacturing, by McKinsey & Company
- Cybersecurity & Data Privacy: An Overview for Health Care, Pharmaceutical, and Biotech Companies, by GIBSON DUNN
- Digital Therapeutics: an exciting innovation, but not without challenges, by Roland Berger
- The AI-driven drug discovery industry: Jury still out on impact, by McKinsey & Company
Newsletter
© INCONCRETO. All rights reserved. Powered by AYM